Laboratory diagnosis of malaria requires the identification of the parasite or its antigens/ products in the patient’s blood.
The requirements of a diagnostic test are specificity, sensitivity, ease of performance and a reasonable cost.
Current available techniques can be separated in three categories:
- Microscopy
- Immunological techniques
- Molecular techniques
1. Microscopy
a) Thick and thin blood smear study
Thick and thin blood smear study is the gold standard method for malaria diagnosis. The procedure follows these steps: collection of peripheral blood, staining of smear with Giemsa stain and examination of red blood cells for malaria parasites under the microscope.
Thick smear. It is not fixed in methanol; this allows the red blood cells to be hemolyzed, and leukocytes and any malaria parasites present will be the only detectable elements. However, the hemolysis may lead to distorted plasmodial morphology making plasmodium species differentiation difficult. Therefore, thick smears are mainly used to detect infection and to estimate parasitemia.
Thin smear. It is fixed in methanol. Thin smears allow the examiner to identify malaria species, quantify parasitemia, and recognize parasite forms like schizonts and gametocytes.
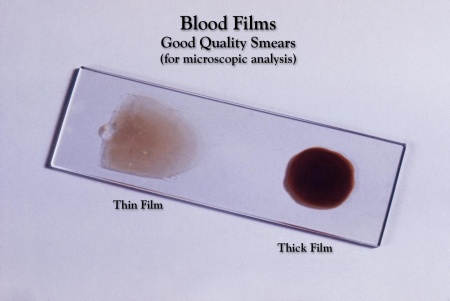
Figure 1 shows how thin and thick smears look like and Figure 2 how a microscopist recognizes the parasites in red blood cells (RBCs).
 |
|
Figure 1. Thick and thin blood smear
(Source: CDC) |
Figure 2. Infected RBCs under microscope
(Source: CDC) |
Advantages:
- It is an inexpensive method
- It gives the examiner the opportunity to quantify parasites and differentiate malaria species
Disadvantages:
- The diagnostic accuracy depends on quality of blood smear and equipment, abilities of the microscopist, parasite density and the time spent on reading the smear. All these may result in therapeutic delays.
- Not suitable for large- scale epidemiological studies .
- False positive. Defective blood film preparation may lead to artifacts that can be incorrectly regarded as malaria parasites. Sometimes, platelets also confound diagnosis.
- False negative. It is associated with low parasite density or low number of fields examined by the microscopist.
b) Quantative Buffy Coat (QBC) test
This method involves centrifuged and compressed red blood cell layer stained with acridine orange and then examinated under an ultra-violet light source. The whole procedure takes place in a glass hematocrit tube which is precoated internally with acridine orange stain and potassium oxalate; it is filled with 55-65 μl of blood. The tube is centrifuged and so the components separate according to their densities forming bands (Figure 3).
Fluorescing parasites are then observed, with a UV microscope, at the red blood cell/white blood cell interface as shown bellow in Figure 4.
QBC test is easier and faster than classic peripheral blood smear microscopy but the equipment required is expensive and species identification and accurate enumeration are impossible.
|
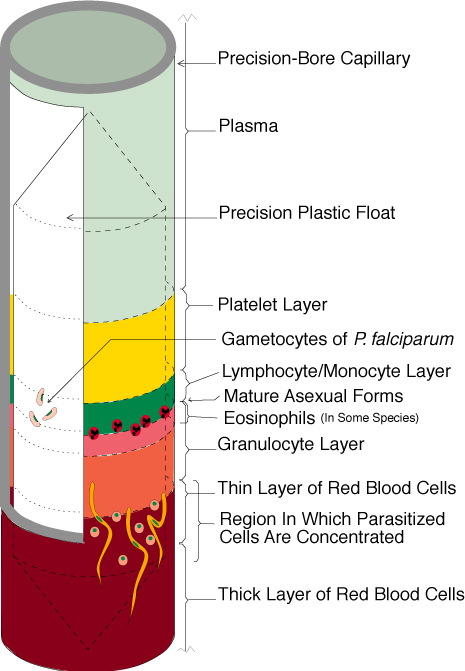 |
Figure 4. Fluorescent parasites under UV microscope
(Source: en.impact-malaria.com) |
Figure 3. QBC, bands formed after centrifugation
(Source: qbcdiagnostics.com)
|
2. Immunological techniques
Antibody-based techniques
a) Indirect fluorescent antibody test (IFAT)
The antigen consists of infected blood bound to a 12-spot microscope slide. A drop of diluted washed infected red blood cells is placed on each spot and allowed to dry. It is then incubated with the serial dilutions of the test serum, followed by a solution of anti-human immunoglobulin labeled with fluorescein isothiocyanine which contains Evans blue as a counterstain. When the slides are dried, they are examined by fluorescence microscopy.
Antibody in the test serum reacts with antigen of parasites and the anti-immunoglobulin reaction with the antibody is demonstrated by the fluorescence of the parasites as shown in Figure 5.
The disadvantages of this method are the requirement of a fluorescence microscope and the need for high technichal skills.
|
|
| |
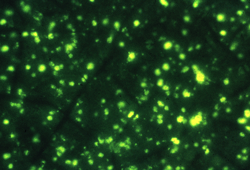
Figure 5. Fluorescence microscopy
(Source: en.impact-malaria.com)
|
b) Enzyme- linked immunosorbent assay (ELISA)
This method uses a soluble malarial antigen coated on the walls of a microtitre plate (Figure 6).
If the test is positive, the antibody binds the antigen resulting in a visible colour change.
When the test is negative, in the absence of antibody, there is no change of colour of the substrate. |
 |
| |
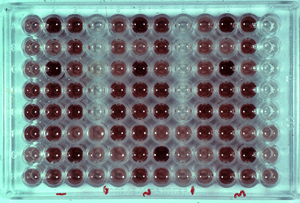
Figure 6. ELISA plate
(Source: en.impact-malaria.com)
|
Antigen-based techniques
Rapid Diagnostic Test (RDT)
RDT is a device that can detect malaria antigen in a small amount of blood (5μl) by immunochromatographic assay (colour change in an absorbing nitrocellulose strip) with monoclonal antibodies directed against the parasite antigen. Depending on the target antigen, rapid tests that now exist may involve combinations of the following: ·
- HRP-2 (Histidine Rich Protein-2) is a protein produced by the asexual stages and gametocytes of P. falciparum, expressed on the membrane of red blood cells (sensitivity: detects parasitemia of >40 parasites/ μl). It often persists in patient’s blood for weeks after successful treatment.
- Plasmodium aldolase is an enzyme of the parasite glycolytic pathway expressed by all malaria species (pan malarial antigen- PMA).
- Lactate dehydrogonase (LDH) is a glycolytic enzyme produced by asexual and sexual stages of parasites and released by infected red blood cells. (sensitivity: detects parasitemia of >100 parasites/ μl)
The PfHRP2 test strips have 2 lines, one for the control and the other for the PfHRP2 antigen.
The PfHRP2/PMA test strips and the pLDH (parasite LDH) test strips have 3 lines, 1 for control, and the other 2 for P. falciparum and non-falciparum antigens.
Change of color on the control line is necessary for the test to be validated. With color change only on the control line and not in the other lines, the test is regarded as negative.
In PfHRP2 test, color change on both the lines is interpreted as a positive test for P. falciparum malaria (Figure 7).
With the PfHRP2/PMA and the pLDH tests, color change on the control line and the pan specific line indicates non-fa1ciparum infection and color change on all the 3 lines indicates the presence of P. falciparum infection (monoinfection or mixed infection with other species) as shown in Figure 7. Therefore, mixed infections ofP. falciparum with non-falciparum species cannot be differentiated from pure P. falciparum infections. |
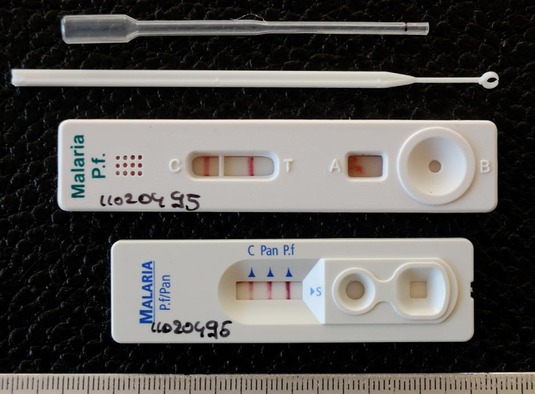 |
| |
Figure 7. Two and three line RDTs positive for P. falciparum or mixed infection |
Advantages:
- Doesn’t require electricity so it can be done on the field distant from microscopy provision
- Simple to perform and to interpret with a result given in 15- 30 minutes
- Ability for outbreak investigation and screening febrile returnees from endemic areas
- HRP-2 tests have sensitivity of> 90% for P. falciparum and LDH tests >95%
Disadvantages:
- Low sensitivity in detecting asymptomatic patients particularly in low parasitemias
- Cross reactions to autoantibodies (such as rheumatoid factor in case of HRP2 test)
- False positivity (gametocytemia, persistent asexual-stage parasitemia below microscopy detection limit)
- False negativity (genetic deletion or mutation of hrp-2 antigen)
In conclusion, a RDT test should always be followed-up by microscopy confirmation of the result; a negative one for excluding low parasitaemia and a positive one for ratification of the species detected in the RDT and for quantifying the proportion of infected RBCs.
3. Molecular techniques
Polymerase Chain Reaction (PCR)
Using PCR amplification, it is possible to detect all 4 species of malaria parasites with a reportedly 10-fold greater sensitivity than microscopy. New technologies such as saponin lysed erythrocytes NAT (nucleic acid amplification technique) and LAMP (loop- mediated isothermal amplification) can provide a lower-cost diagnosis with greater sensitivity and specificity for the 5 plasmodium species accepted by WHO; although they may become useful in detecting plasmodium infections in healthy adults with “submicroscopic” levels of parasitemia, there is still no PCR test approved by the Food and Drug Administration for the diagnosis of malaria or blood donor screening and further development should follow in order for these methods to be widely applied as diagnostic tests.
Advantages:
- High sensitivity and specificity
- Detection of mixed species infections and drug- resistant strains
- Automation, high speed turnover
- Quantitative determination of parasite and species differentiation ability
Disadvantages:
- Too sensitive for clinical use
- Unsuitable for field conditions
- Expensive
- Technically demanding